What are 6 health problems that might be treated by stem cells?
WHAT ARE 6 HEALTH PROBLEMS THAT MIGHT BE TREATED BY STEM CELLS?
Stem cells are compleletly or partially undifferentiated or unprogrammed cells which are capable of developing into numerous types of cells and serving different functions in different parts of the body. The majority of cells in the human body are differentiated cells which means that they can serve only a specific purpose in a particular organ such as red blood cells that can only carry oxygen or carbon dioxide with the blood.
Stem cells can divide and make an indefinite number of copies of themselves. Stem cells can either remain stem cells or turn into differentiated cells such as red blood cells or muscle cells.
How stem cell could be useful for treating different diseases.
Scientists involved in stem cell research believe that stem cells can be useful for understanding mechanisms of diseases and treating those. Stem cells are being used to:
- Replace damaged cells of organs or tissues,
- Resore functions of tissues or organs that do not work properly,
- Investigate mechanisms of genetic defect caused diseases,
- Research how diseases develop or why certain cells become cancerous,
- Test safety and effectiveness of new drugs.
The stem cell field has grown rapidly over the last two decades with stem cell treatments that include technologies to treat or prevent a disease condition. Stem cells have the potential to help treat numerous diseases by generating healthy new cells of almost all tissues.
Stem cell therapy for treating hematopoietic and genetic disorders
Stem cell therapy is being used to treat hematopoietic and genetic disorders. For instance in a cord blood transplant, stem cells are infused into a patient’s blood stream after which they help in healing and repairing of damaged tissues. Upon successful engraftment of the stem cells, the patient’s blood and immune systems are regenerated.
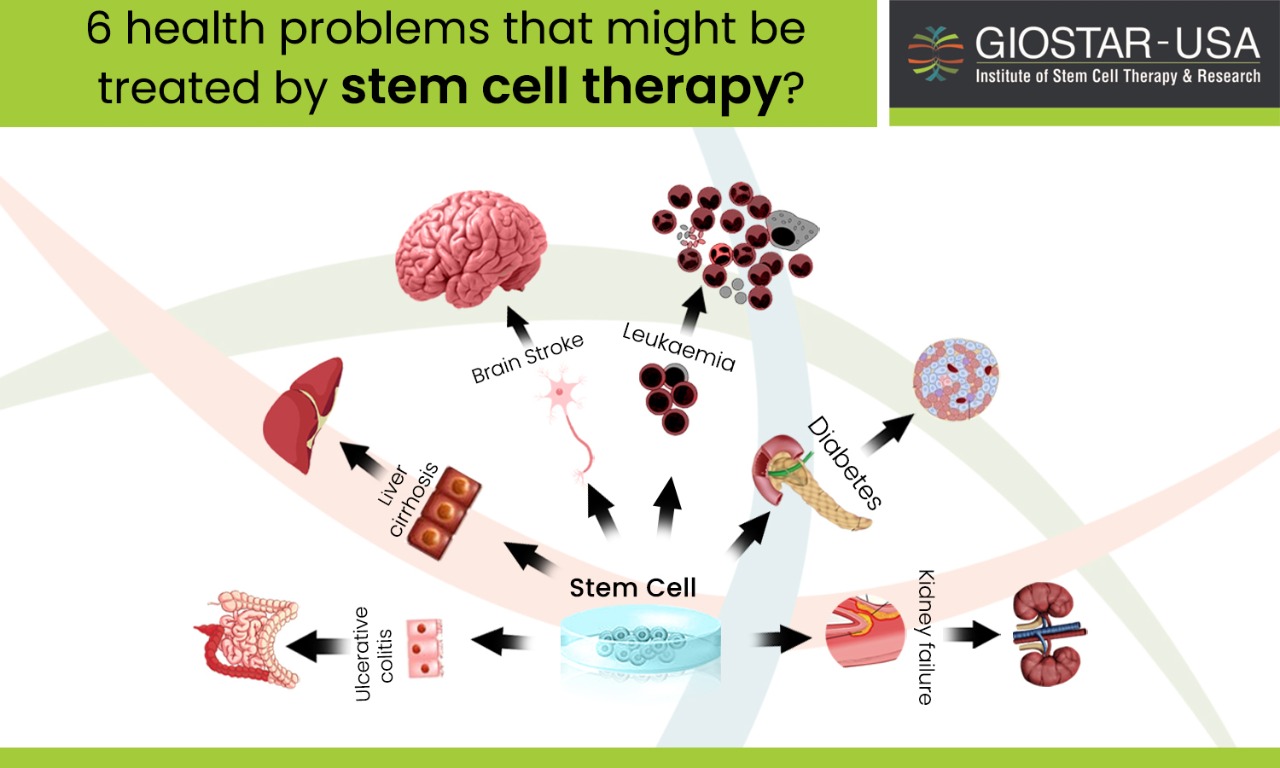
Six Health Problems that might be treated with stem cell therapy-
There are a wide range of diseases that are treatable with stem cells derived from cord blood or from other sources like bone marrow and peripheral blood. The diseases treatable by stem cells include acute and chronic forms of leukemia, myeloproliferative disorders, and many more.
The following six health problems might successfully be treated by stem cell therapy also known as regenerative medicine-
- Leukaemia
Doctors are replacing cells damaged by chemotherapy or leukemia by performing stem cell transplant. Stem cell therapy is also serving as a way for supporting the patient’s immune system to fight some types of cancer and blood related diseases.
- Diabetes – type 1
Embryonic stem cells when transplanted into people with type 1 diabetes is showing some very encouraging results towards the management of this disease.
- Stroke
Stem cells are being used in clinical trials with evidence that when combined with clot busting or mechanical thrombectomy therapy enhances recovery. Stem cells injected into distant arteries or veins travel to the site of stroke in the brain fueling the repairing and recovery processes.
- Kidney failure
Mesenchymal stem cells when administered independently are known to aid in kidney repair in cases of kidney failures.
- Liver cirrhosis
The mechanism of mesenchymal stem cells or MSCs for treating liver cirrhosis /diseases has been evaluated from various perspectives. Adult stem cell therapies help induce the rapid production of hepatocytes in patients with liver cirrhosis.
- Ulcerative colitis
Mesenchymal stem cells have displayed their immense potential for suppression of overactive immune response and for healing inflamed tissues. Therefore, these are emerging as a promising treatment of ulcerative colitis and inflammatory bowel disease or IBD, the diseases suspected to be rooted in localized inflammation in digestive tract.
The stem cell therapy has great potentials for treating and/or managing the six diseases mentioned above.